Mobility Database
PanMg2024_MB is an atomic mobility database for Mg-based alloys, which is compatible with the PanMg2024_TH thermodynamic database and suitable for the simulation of diffusion-controlled phenomena using the PanDiffusion module, PanEvolution module, and/or PanSolidification module.
Phases
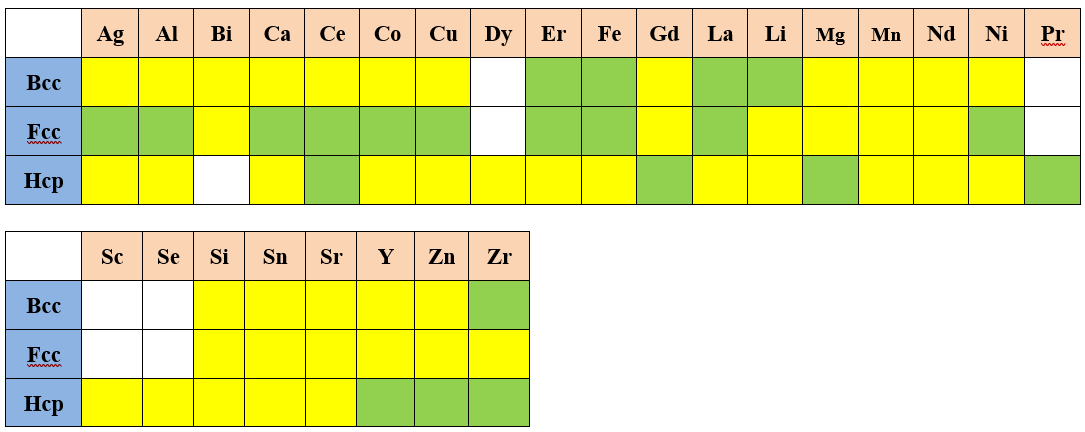
The atomic mobility within the Liquid, Bcc, Fcc, and Hcp solution phases are assessed in this database.
Self-diffusivity of Pure Elements
The color represents the following meaning:
| : | Validated | |
| : | Estimated | |
| : | No data |
Assessed Systems
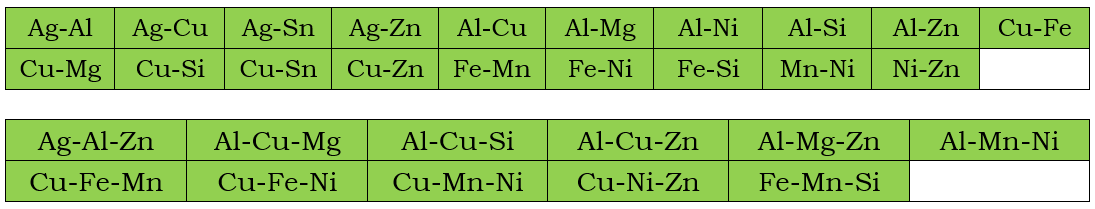
In addition to the assessed self-diffusivities shown above, the impurity diffusion data for all included elements in the current PanMg2024_MB database are also assessed. In the following, the assessed chemical-diffusivity within the binary and ternary systems for the Fcc, and Hcp phases are listed, respectively.
Fcc Phase
Hcp phase
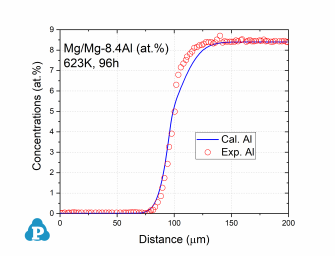
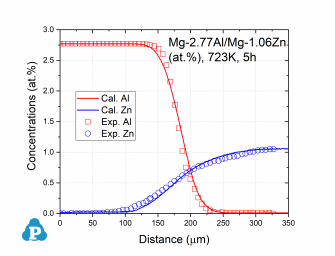
Database Validation
The simulated concentration profiles of a series of magnesium alloys are used to validate the current mobility database for Mg-based alloys. A few examples of such simulation are shown below.
Applications
This mobility database is combined with the thermodynamic database for Al-based alloys, PanMg_TH, to simulate the diffusion-controlled phenomena of Mg-based alloys. A few examples are given below.
Precipitation kinetics of magnesium alloys
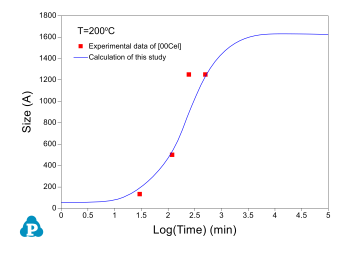
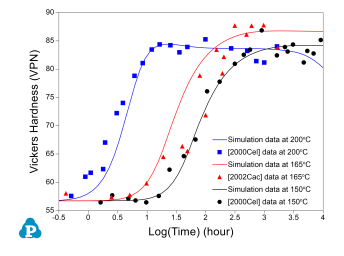
The PanEvolution module was developed for the simulation of precipitation kinetics of multi-component alloys. It has been seamlessly integrated with the thermodynamic calculation engine of Pandat™ software, and has been used to simulate the evolution of microstructure and the corresponding mechanical property responses to heat treatment magnesium alloys [2011Cao]. Below shows an example simulation performed for the AZ91 alloy. Figure 3 shows the simulated particle size evolution with time of the γ-Mg17Al12 precipitate aged at 200 °C compared with the experimental data of [2001Cel]. Figure 4 shows yield strength evolution with time. As is seen, the particles grow and coarsen with ageing time, while the yield strength reaches peak at a time varies with heat treatment temperature. The database used to do this simulation is the combined thermodynamic and mobility database of Mg-based alloys: PanMg_TH+MB. More information regarding to precipitation simulation can be found in PanEvolution module under the Software section.
Figure 3: Calculated particle size evolution of the γ-Mg17Al12 precipitate aged at 200 °C compared with the experimental data of [2001Cel]
Figure 4: Simulated hardness curves of AZ91 alloy aged at 150, 165 and 200 °C compared with the experimentally measured data of [2001Cel, 2002Cac]
Solidification of magnesium alloys
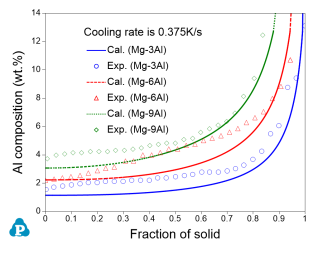
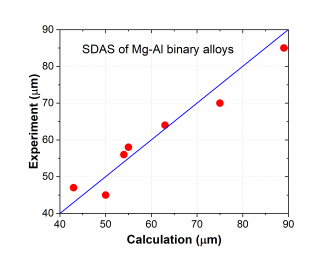
The combined thermodynamic and mobility database of Mg-based alloys: PanMg_TH+MB is also used to simulate the solidification process considering back diffusion in the solid phase [2019Zha]. Figure 5 compares the simulated and measured [2013Pal] Al composition profiles in the α(Mg) phase vs. fraction of solid (fs) for Mg-xAl (x=3, 6 ,9) alloys. Figure 6 compares the simulated and measured secondary dendrite arm spacing (SDAS) [2013Pal2] results of Mg-Al binary alloys. More information regarding to solidification simulation can be found in PanSolidification module under the Software section.
Figure 5: Comparison between the simulated and measured [2013Pal] Al composition profiles in the hcp_α(Mg) phase vs. fs for Mg-xAl (x=3, 6 ,9) alloys
[2001Cel] S. Celotto and T.J. Bastow, Study of precipitation in aged binary Mg–Al and ternary Mg–Al–Zn alloys using 27Al NMR spectroscopy. Acta Materialia, 49(1) (2001): 41-51.
[2002Cac] C.H. Caceres, Hardness and yield strength in cast Mg-Al alloys. AFS Transactions, 110 (2002): 1163-1169.
[2011Cao] W. Cao, et al., An Integrated Computational Tool for Precipitation Simulation. JOM, 63(7) (2011): 29-34
[2013Pal] M. Paliwal, Microstructural development in Mg alloys during solidification: an experimental and modeling study, in Department of Mining and Materials Engineering 2013, McGill University: Montreal, QC.
[2013Pal2] M. Paliwal and I.-H. Jung, The evolution of the growth morphology in Mg–Al alloys depending on the cooling rate during solidification. Acta Materialia, 61(13) (2013): 4848-4860.
[2014Kam] C.C. Kammerer, et al., Interdiffusion and impurity diffusionin polycrystalline Mg solid solution with Al or Zn. Journal of Alloys and Compounds, 617 (2014): 968-974.
[2016Kam] C.C. Kammerer, et al., Interdiffusion in Ternary Magnesium Solid Solutions of Aluminum and Zinc. Journal of Phase Equilibria and Diffusion, 37(1) (2016): 65-74.
[2019Zha] C. Zhang, et al., CALPHAD-Based Modeling and Experimental Validation of Microstructural Evolution and Microsegregation in Magnesium Alloys During Solidification. Journal of Phase Equilibria and Diffusion, 40(4) (2019): 495-507.